
This is a question landing on our helpdesk very often and although I have written about this question in different blog posts, Instagram carousels and in cosmetic preserving courses, I decided to give a short wrap up for the young generation who doesn't have the time and patience to go shovel for old blog posts and information.
First things first: What are glycols?
Glycols are a broad range of molecules almost indispensible in our everyday life. As a chemical group, they lie between alcohols and glycerol. Alcohols are organic molecules with one (-OH) group dangling from a hydrocarbon skeleton. The most familiar alcohols are: ethanol (the one found in distilled and brewed alcoholic drinks as well as the one we use for disinfecting), n-propanol and iso-propanol (these are solvents and disinfecting ingredients).
Ethylene glycol, the first one in the homolog series and the first one ever synthesized was first prepared in 1859 by the French chemist Ethylene glycol was first prepared in 1859 by the French chemist Charles-Adolphe Wurtzt. He adopted the name "ethylene glycol" because he has observed that this molecule has properties between ethyl alcohol and glycerol.
Although ethylene glycol was first prepare in 1859, there were no industrial manufacturers or application for it till the first wolrd war. It means glycols are commercially on the market since about 150 years. They are available to affordable prices and are manufactured in huge volumes.
In contrast to glycerine and ethyl alcohol however, the glycols are not found in nature. They are totally synthetic. Now till about 2 decades ago glycols were synthesized in huge volumes and at a very affordable price from petrochemicals. With the increasing demand for a greener chemistry and plant derived ingredients, glycols are now being derived from maiz or sugar cane (obviously at a much higher price).
The NI of the plant derived glycols is 0 according to Iso 16128 and the NOI is 1.
Ethylene glycol is a highly toxic chemical. It is used as antifreeze and coolant in motors. In fact its breakthrough was during teh world war I when it was used as a coolant for fighter plane motors.
The next molecule in the homolog series is the propylene glycol with 3 carbon atoms. This one is a safe chemical and is ubiquitous and you can find it almost everywhere from mouthwash and toothpaste to ice cream, food, beverages, industrial cleaners and anti-freeze (that might sound eeky to have a bit of anti-freeze in your mouthwash or in your ice cream).
It is a colourless and odourless liquid with a mild sweet taste. It reduces the freezing point of water and hence is used in anti-freeze as well as in frozen dairy and icing. It is added to food and beverages as well as in alcoholic drinks as a fragrance enhancer, solubilizer, viscosity and texture modifier. It is much more safer than ethylene glycol because it is metabolized to lactic acid in body but in internal application it is not quite considered safe in high dosages. In Europe its application dosage is highly controlled because PG can cause liver, respiratory, and kidney dysfunction, especially in children or adults with existing liver or kidney malfunction.
In 2014 Fireball whiskey was recalled from 3 European markets because they've accidentally shipped the whiskey designed for the American market to the Europe. The high propylene glycol content in the whiskey was in limit for the American market yet much higher than the European limits.
As the chain becomes longer and longer (C4, C5, etc.) the glycols are still humectants, solvents, stabilizers. Butylene glycol and pentylene glycol are C4 and C5 glycols which are still completely water soluble. From hexylene glycol, C6, the molecules become insoluble in water. (C6 and C7 are still slightly miscible with water).
Why do we use them?
Glycols are used because of their neutral (slightly sweet) scent and neutral colour. Their excellent chemical stability and their long shelf-life.
Propylene glycol is a much more superior solvent to glycerine and hence is a main component of most hydrophylic conventional plant extracts . In water based products glycols improve the stability of the product at lower temperatures and improve solubility of certain ingredients.

In micellar waters, tonics and splashes (products with a high water content) for example, pentylene glycol is added to improve the stability of the product at lower temperatures (improving the freeze/thaw stability).
Glycols are no preservatives on their own. In the EU the preservatives allowed in cosmetic products are listed in Annex V of the cosmetic directive. This list includes 60 chemicals that are accepted as cosmetic preservatives.
Glycols however can boos preservative efficacy and some of them are blended with commercial preservatives to enhance the performance.
How they work
Check this carousel for more information about the challenge test in the EU and the products that are exempt from the challenge test.

Like glycerine, glycols reduce the water activity. Water activity is a term used in physics which refers to "available water" instead of the concentration of water.
This term is extremely useful in preservation. Not only in food and beverages but in construction industry, pharmaceuticals and cosmetic industries.
aw = P/Po = (n2/(n1 + n2))
It is the ratio of the water vapor pressure of the product compared to pure water at the same temperature, where P is the vapor pressure of the product, Po is the vapor pressure of pure water, n1 is the number of moles of solute, and n2 is the number of moles of water (bless me, if you are not a chemist, this all seems like a foreign language to you but don't worry you don't need to learn this by heart).
Available water or water activity is the content of water that is available to microorganisms for survival and growth and each microorganism has its min. required water activity for survival.
The aw is not the same as the content of water. Honey for example has a high content of water but the water activity is so low that honey is self-preserving despite the high content of water. A loaf of bread in contrast has a much lower water content but the water activity is high enough for molds to grow and hence you can not keep a loaf of bread for a long time without mold happily growing on it.
There are commercial instruments available for measuring water activity and they are very common in the food industry (for grains, dried nuts and fruits, pasta etc.) which are all working based on the equation mentioned above.
Now reducing the water activity is not a modern "thing" and our ancestors did it to preserve the food all the time but didn't have any fanciful name for that.
Using sugar and salt or drying (smoking) food was a safe way to preserve food long before refrigerators were available.
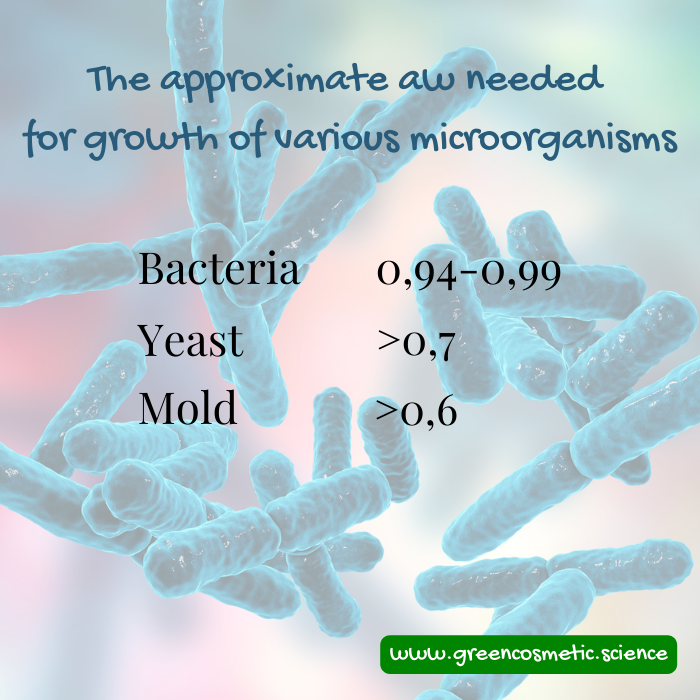
In the following chart you'll see the growth requirements for bacteria, yeast and mold. It means, when the water activity is lower than 0,6 the product (food, cosmetic, cement, paint etc) is safe against contamination.
Here you can see the water content of a few common cosmetic products. In water containing formulations, the water activity is high enough for all microorganisms to happily live and grow there. This is why most cosmetic formulations need an effective preservative system.

In the EU, a product is considered self-preserving and is exempt from challenge test when the water activity is lower than 0,75.
As you can see, all water containing formulations have a much higher water activity than the max. required for self-preserving.
In the following chart you can see the water activity required for most common cosmetic contaminants.

So coming back to the glycols and the question whether they are preservatives.
Glycerin and glycols have preserving properties by reducing the water activity.

The min. concentration however is too high for them to be practical in a cosmetic formulation with an applealing sensory properties.
Butylene glycol and the glycols with a higher carbon chain are used however as preservative boosters and usually when you use them, you can reduce the preservative concentration (this all needs to be backed up by challenge testing).
That brings us to the main question and topic of this post:
Pentylene glycol is NOT a preservative but can be used as a preservative booster.
However, it can be used as the main solvent for botanical extracts and infusions and usually you don't need to add any preservative (again, you need to back up the theory with challenge testing). Apart from that, it is used to preserve hydrosols (a concentration above 5%). In formulations such as toners and micellar waters with a low concentration of surfactants and fat, it may be efficient at a concentration above 5% as a preservative by reducing the water activity (this depends on the formulation, packaging , application mode and the quality of raw material and must be backed up by challenge testing).
May all your products remain safe from contamination.


