
Plant extracts are indispensable in modern cosmetics specially in natural cosmetics. Making your own extracts is one of the most fun parts of making plant based cosmetics specially if you have access to high quality herbs and plants.
In modern, herbal based cosmetic formulation the number of the carriers you can choose for extraction are rather limited. In making drugs and pharmaceuticals as well as in research and analytical labs the main concern is to EXTRACT individual components with the highest possible yield and neither safety nor environmental concerns are dominant when it comes to choosing a solvent/carrier. When you refer to scientific articles you see they are using solvents that make your toes curl but these solvents, as effective as they might be, have no place in ecological, environmental friendly and safe modern cosmetics.
Glycerine, alcohol, water and combinations of them as well as plant oils have been used by herbalists and alchemists for centuries for macerating plants and using them in balms, compresses and concoctions. The number of available safe and ecological solvents has not changed much today except for the addition of glycols which are excellent hydrophilic/polar solvents. Glycols are available both as natural and plant based ingredients and as mineral oil based. It makes sense to use the plant based ones (which are much more expensive than the synthetic ones) for herbal extraction if you are making "natural" and environmental friendly products. Mineral oil based glycols are synthesizing and are not used at high concentrations in cosmetics whereas the plant based ones are skin friendly and cause no sensitization.
When it comes to making herbal extract, apart from the fun and the therapeutic effect of packing jars with plant material and filling them with a solvent, the main question that you need to ask yourself is: What do I want to extract?
This seems a simple and primitive question but many novices jump right to the extraction part without considering this question and thinking about the components that they plan to extract. If you are, like the mainstream industry, just looking for a name on the label or the beauty of the filled jars with plants and solvents then in that case it really doesn't make much difference what you are using but if you are aiming to extract some useful components then it makes a huge difference which solvent you choose and use. The solvent/carrier not only predestines which components you can extract but also how and where you can use the extract as well as the shelf-life of the extract.
We have dedicated several posts in the past to this subject and you can have a look at the following posts for a detailed explanation about extracts, solvents and extraction methods:
Welcome into the labyrinth of plant extracts (part I)
Welcome into the labyrinth of plant extracts (part II)

Extraction methods
From a technical point of view there is a difference between a maceration/infusion and an extract. Extracts are usually made by crushing the plant cells either through mechanical/thermal force or microwave or ultrasound. Obviously when the plant cells are crushed the yield of the active material is much higher and most industrial extracts are made this way.

Although these methods have a much higher yield, the necessary filtration process will be a huge headache and not really feasible in an artisanal lab. It is not quite impossible but it is so tiresome that kills the whole joy of making and using the extract before the extract is even ready.
This is why herbalists and most artisanal formulators (and even some industrial suppliers) rather use infusions/macerations instead of real extracts. In an industrial scale and when working with low boiling point solvents such as acetone, alcohol or hexane, they use percolation as an alternative extraction method. In percolation, the solvent passes through the plant material, extracts a certain amount of active ingredients, then is recollected by distillation and reused for another cycle. This procedure allows efficient extraction with a low quantity of solvent but is very energy consuming and is not efficient with solvents such as glycols, glycerine or plant oils. In our artisanal labs, let's stay by infusion/maceration and environmental friendly solvents.

In an artisanal scale the main classification of the solvent divides them into:
1- Lipophilic solvents
2- Hydrophilic solvents
Each group of these solvents have several possible options for a "natural cosmetics" concept and you can choose one or a blend of several solvents/carriers
considering:
- The price
- The availability
- Thermal stability and shelf-life
- Polarity
- Impact on the scent and color of the extract/product
As lipophilic solvents you can use any plant oil with a long shelf-life and reasonable thermal stability. Obviously you will not choose borage oil or evening primrose oil because they have a very short shelf-life and are rancid before even your extract is ready. Sunflower oil and rapeseed oil are the most commonly used lipophilic carriers because of their availability and rather affordable price as well as being neutral in scent (the deodorized rapeseed oil has next to no scent in contrast to the native oil) and color. Herbalists sometimes use jojoba oil, camelina oil or even sesame oil. When you are making your own extracts having a certain product and concept in mind, you have the advantage of choosing the solvent that matches your product formulation and concept the best.
In an upcoming blog post we will show you how one of our members has used squalane for a lipophilic maceration. Squalane is much more expensive than most plant oils and has a lower polarity compared to plant oils but has an outstanding thermal and oxidative stability and does not interfere with the scent or color of the extract.
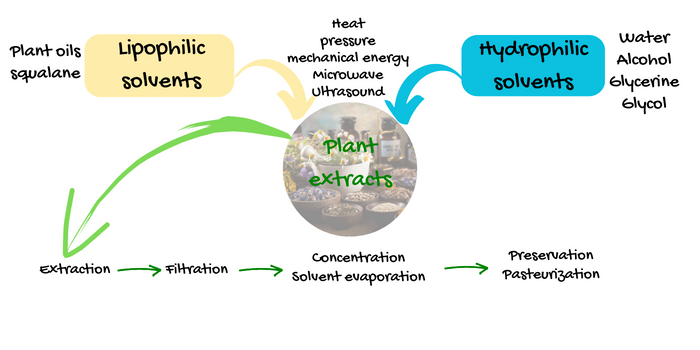
As hydrophilic solvent/carrier water is the most affordable and cheapest ones and is the solvent often used by herbalists for infusions and compresses. When it comes to cosmetic formulations however, water is not a suitable carrier. Because of its high polarity however it is often used in mixture with other hydrophilic solvents.
Keep in mind that aquous extracts are extremely difficult to preserve. Apart from that they do not have a reasonable stability when it comes to scent and color. If you are making a product for your own home use you certainly can use water as the solvent (boiling water before extraction and preserving the product will eliminate possible contaminants) but for a commercial product with a reasonable shelf-life water is a poor solvent. In the food and beverage industry and sometimes even in the cosmetic industry water based extracts are pasteurized to eliminate the risk of contamination and the need for preservation.
Alcohol which is often used by herbalis and in pharmaceutical extracts either alone or in various combinations with water is not a suitable carrier in cosmetic formulations. Alcoholic and hydroalcoholic industrial extracts are often concentrated to reduce the content of the solvent or are completely dried to powders. Powder extracts have a low water solubility, so if your formulation doesn't contain any alcohol, whichit usually does not, the powdered extracts are next to useless for you.
Glycerine and glycols are among other hydrophilic solvents/carriers. They are both, self preserving as pure carriers but when blended with water the extracts need an efficient preservative system.
Glycerine is a much poorer solvent compared to glycols but because of its availability and relatively cheap price it is very often used for extraction. They are both available as synthetic and mineral oil based ingredients as well as natural and plant based ones. Obviously if you are working in a "natural" or "environmntal friendly" concept you will coose the plant based versions.
The sol called "glycerites" are extremely popular among DIYers in which the plant material (usually colorful fruits) are macerated in glycerine and then filtered after a short while. These glycerites are usually very pretty in color and sometimes you can even catch some volatile material which contribute to a scent but they are extremely unstable. Apart from that applying a high dosage of glycerine in a finished formulation usually contributes to tackiness and an unpleasant skin feel. Glycolic and hydroglycolic extracts/macerations have a superior stability and in contrast to glycerine, glycols have a much more pleasant and less sticky skin feel.

Plant material usually have a high load of contaminants. When you dry the plant parts it reduces the water activity and eliminates the possibility of growth but it doesn't eliminate the number of existing microorganisms all together.
Even making a lipophilic extract does not mean that your extract is free from contaminants. In fact Andry, the author of our upcoming blog post about plant extracts has prepared a plant extract in squalane and sent it to the lab and the results were so that the extract needed to be prepserved for safe formulations.
Although reducing water activity is a kind of self-preserving and theoretically and legally a waterless product does not need a challenge test we have observed that some safety assessors demand a challenge test when there are lots of plant extracts present in a waterless product. This means that if you are preparing an extract to be used in a comercial product you may need to have it tested for the microbial count and you may need to preserve that extract.
Hydroglycolic and hydroglyceric extracts inherently need a preserving system. Some industrial suppliers however apply specific sterilization techniques that exempt the extract from microbial testing and preservation,
Obviously the preservative system you are using must be compatible with the formulation concept you are going to use the extract in it. Making your own extracts has the advantage that you can design the extract and apply a suitable preservative system right from the beginning.
In the following post Andry from Jacinthe Naturals will show you how she has prepared a lipophilic nettle extract and share her laboratory test results.


